Astetica
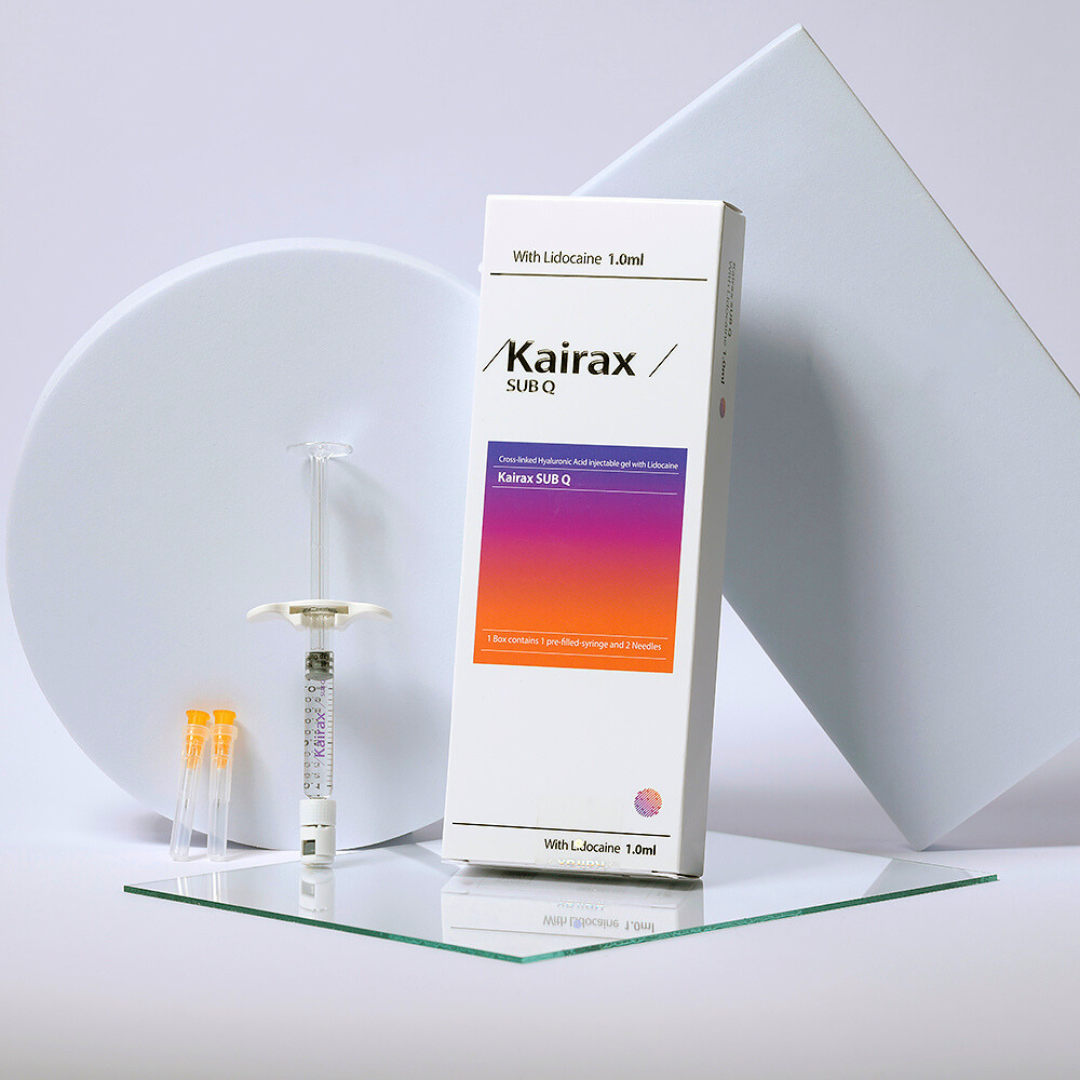
Kairax Sub-Q (1 × 1.1 mL)
Kairax Sub-Q (1 × 1.1 mL)
Couldn't load pickup availability
Clinical Summary
Kairax Sub-Q is a cross-linked hyaluronic acid dermal filler containing lidocaine, formulated with high viscoelasticity for deep volumisation and structural contouring.
Key Benefits
- Highest viscoelasticity within the Kairax range for deep volume support
- Contains lidocaine (0.3%) for enhanced patient comfort
- CE-marked hyaluronic acid dermal filler
- Hydrophilic HA supports moisture retention and tissue integration
- Supplied in a sterile, ready-to-use pre-filled syringe
Indications / Use Cases
Indicated for deep volumisation and contouring procedures, including facial structure enhancement and selected body contouring applications, when administered by trained professionals.
Product Specifications
- Volume / Strength: 1.1 mL / 20 mg/mL hyaluronic acid
- Pack Size: Box of 1
- Presentation: Pre-filled sterile syringe
- Needles Included: 1 × 25G needle, 1 × 27G needle
- Lidocaine Content: 0.3%
- Duration: 6-12 months
- Storage: Store at 2–25°C
- Manufacturer: Thepharm
- Country of Origin: South Korea
Treatment Areas
- Chin
- Jawline
- Nose
- Cheeks
Contraindications
Kairax Sub-Q should not be used in:
- Women who are pregnant or breastfeeding
- Individuals under 18 years of age
- Patients with known hypersensitivity to hyaluronic acid
- Patients with known hypersensitivity to lidocaine
- Patients prone to hypertrophic scarring
- Areas with active inflammation or signs of infection
- Patients undergoing cancer treatment
- Immediately before or after laser therapy, chemical peeling, or dermabrasion
Professional Use Disclaimer
This product is supplied for use by qualified healthcare professionals only. It is not intended for sale to the general public. Always use in accordance with the manufacturer’s instructions, applicable clinical guidance, and local regulations.
Regulatory / Prescription Notes
This product is intended for administration by trained aesthetic practitioners only. Professional status verification may be required prior to supply.